TLDR photosynthesis converts sunlight, water, and CO2 into energy. Animal cells consume oxygen and sugars and emit CO2. By creating animal cells that consume less oxygen and emit less CO2, we can defeat the disgusting Indians and Chinese raping the planet once and for all.
Abstract
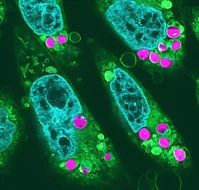
Chloroplasts are photosynthetic organelles that evolved through the endosymbiosis between cyanobacteria-like symbionts and hosts. Many studies have attempted to isolate intact chloroplasts to analyze their morphological characteristics and photosynthetic activity. Although several studies introduced isolated chloroplasts into the cells of different species, their photosynthetic activities have not been confirmed. <mark>In this study, we isolated photosynthetically active chloroplasts from the primitive red alga Cyanidioschyzon merolae and incorporated them in cultured mammalian cells via co-cultivation.</mark> The incorporated chloroplasts retained their thylakoid structure in intracellular vesicles and were maintained in the cytoplasm, surrounded by the mitochondria near the nucleus. Moreover, the incorporated chloroplasts maintained electron transport activity of photosystem II in cultured mammalian cells for at least 2 days after the incorporation. Our top-down synthetic biology-based approach may serve as a foundation for creating artificially photosynthetic animal cells.
Article
In an incredible feat that redefines biological boundaries, scientists have successfully engineered animal cells capable of photosynthesis. This breakthrough, led by Professor Sachihiro Matsunaga at the University of Tokyo, could transform medical research and aid in advancing lab-grown meat production. Photosynthesis, traditionally exclusive to plants, algae, and certain bacteria, is a process that uses sunlight, water, and carbon dioxide to produce oxygen and sugars – essentially "feeding" the organism. "All living organisms on Earth, including humans, are able to live thanks to photosynthesis," Matsunaga said. "Animal cells consume oxygen, eat and break down sugars, and emit carbon dioxide. This reaction is completely opposite to photosynthesis." By introducing photosynthetic properties into animal cells, the team hopes to create cells that consume less oxygen and emit less carbon dioxide – essentially turning them into mini oxygen producers.
Attempts to create photosynthesizing animal cells date back to the 1970s. <mark>"If we can get even part of photosynthesis to occur in animal cells, we can reduce the amount of oxygen consumed, reduce the amount of sugar eaten, and reduce carbon dioxide emissions," Matsunaga said.</mark> However, the challenge lies in convincing animal cells to accept chloroplasts, the cellular structures where photosynthesis happens in plants. Previously, animal cells would destroy chloroplasts, recognizing them as foreign invaders, like bacteria or viruses. After a decade of unsuccessful attempts, research in this field was abandoned, and it became widely accepted that chloroplasts could not function within animal cells.
Matsunaga's team took two major steps to achieve this scientific breakthrough. First, they searched for chloroplasts that could survive the warmer environment of animal cells, which are usually cultured at around 37 degrees Celsius – significantly higher than the temperatures that most plant chloroplasts can endure. After finding suitable chloroplasts, the team had to prevent animal cells from rejecting them as foreign material. "Chloroplasts eaten as food could be maintained in the animal cell for at least two days, during which time the initial reaction of photosynthesis could be detected," said Matsunaga. By allowing animal cells to ingest chloroplasts as "food" rather than forcibly implanting them, the team managed to bypass the immune response that typically destroys the chloroplasts.
The initial results surprised Matsunaga and his colleagues. Not only did the animal cells tolerate the chloroplasts, but they also showed an increased growth rate, suggesting that the chloroplasts provided an additional energy source. "I was surprised because we were able to do something that no one had been able to do for 50 years and that all biological researchers had given up on," Matsunaga said. This achievement may have broad implications, particularly for medical applications and artificial meat production.
While human photosynthesis remains a distant goal, Matsunaga believes the immediate applications lie in medical research and lab-grown meat production. One major obstacle in growing tissue for medical use or artificial meat is ensuring oxygen delivery to densely packed cells. As Matsunaga explained, "when cells become multilayered, the interior of the cell mass [doesn't get enough oxygen]; cell division stops, and size increase is not possible." By embedding chloroplasts, these cell clusters can generate their own oxygen if exposed to light, potentially resuming cell division and growth. Looking further into the future, Matsunaga envisions medical applications that would deliver oxygen to specific organs, such as the heart. For instance, implanting a small LED near the heart could provide light to photosynthetic cells, improving oxygen delivery in heart disease patients. However, extending the chloroplasts' lifespan within animal cells remains a challenge. "In the future, we will improve our technique so that chloroplasts can carry out photosynthesis in animal cells for as long as possible," Matsunaga said.
Matsunaga's study marks a paradigm shift, showcasing how engineering animal cells to photosynthesize could lead to numerous innovations, from lab-grown meat production to enhanced medical treatments. While there is still work to be done to extend chloroplast function within these cells, Matsunaga's team has opened the door to a new realm of possibilities, challenging long-standing beliefs in biology and pushing the boundaries of what cells can achieve. This breakthrough underscores the transformative potential of photosynthesis beyond plant life, offering a unique and versatile tool for addressing human challenges in food production, medicine, and possibly even climate change. The study is published in the journal Proceedings of the Japan Academy, Series B.









Jump in the discussion.
No email address required.
So how soon can we start introducing chloroplasts into human cells?
Jump in the discussion.
No email address required.
More options
Context